|
Free Trial
-
Explore 20 selected sample compounds with our CC-DPS information available for free.
-
For free trials with your chosen compounds, visit the CC-DPS processing site and submit them. No charge for compounds with up to 5 non-hydrogen atoms.
Subscribe to Mol-Instincts
-
Unlock instant access to over 10 billion datasets for 5 million chemical compounds, each with more than 2,100 sets of CC-DPS information.
-
For paid subscribers, we offer fully free CC-DPS processing for any compounds not available in Mol-Instincts.
-
Discover the perfect subscription plan for your needs by contacting us at contact@cc-dps.com.
About
First Chemical Database Based on Quantum Chemistry
5+ million chemical compounds
Database for chemical property information
Cited in Research Papers Like Nature
Based on 41 Patented Technologies
Awarded Korea Best Patents for 2014
The World's Largest in terms of Information Volume
Developed NAVER Chemical Structure Dictionary
What is Mol-Instincts?
-
NEW
A New Chemical
DatabaseWorld’s First chemical database based on quantum chemistry.
-
5+Million
More than 10 Billion
Sets of Data and InfoOver 2,100 sets of data are available for each and every 5+ million compounds.
-
95%
High Level of Accuracy
The level of prediction accuracy by Mol-Instincts has been verified to be above 95% in most cases when compared with experimental data available to date
(other existing methods, e.g., Joback method provides 63% of the accuracy level for boiling point prediction)
Product Summary
World’s First Database Based on Quantum Chemistry and QSPR with 41 Related Patented Technologies.
Physical properties of chemical compounds are usually determined by experimental methods, which are time-consuming and costly to perform. In addition, in many cases experimental techniques are impossible to do due to the impurity, toxicity, and instability of chemicals.
Mol-Instincts has been developed based on 41 patented technologies, combining quantum chemistry, fundamental scientific methods, statistical thermodynamics, QSPR, SVRC, and ANN with a proprietary over-fitting prevention algorithm. Thanks to the automatized Mol-Instincts technologies, chemical properties can be analyzed within 10 hours while having the advantage of being affordable costing under two dollars.
-
Quantum Chemical Calculation, QSPR Molecular Descriptors
-
40 Patented Technologies
Fundamental Scientific Approaches
Statistical Thermodynamics
QSPR (Quantitative Structure-Property Relationships)
SVRC (Scaled Variable Reduced Coordinates)
Over-fitting Prevented ANN (Artificial Neural Network) -
Quality Inspection of Predicted Data
-
1 Patented Technology
Exclusive Compounds
| Hydrocarbons | 958,000+ | |
|---|---|---|
| Nonhydrocarbons | Hetero Compounds | 1,510,000+ |
| Halogen Compounds | 50,000+ | |
| Extra-Hetero Compounds | 10,000+ | |
| Drug-like Compounds | 1,312,000+ | |
| Fuel Compounds | Gasoline | 105,000+ |
| Jet-Fuel | 171,000+ | |
| Diesel | 735,000+ | |
| Biodiesel | 672,000+ | |
| Chemical Processes | Soot Aromatic | 248,000+ |
| Naphtha | 273,000+ | |
| Combustion | 1,349,000+ | |
| Thermal Cracking | 491,000+ | |
| Catalytic Reforming | 408,000+ | |
| Catalytic Cracking | 798,000+ | |
| Hydro Cracking | 768,000+ | |
| Desulfurization | 1,012,000+ | |
| Isomerization | 231,000+ | |
| GTL (Gas-To-Liquid) | 858,000+ | |
| CTL (Coal-To-Liquid) | 1,249,000+ | |
| MTO(Methanol-To-Olefin)/ MTG(Methanol-To-Gasoline) |
689,000+ | |
Categories & Applications
Molinstincts Information & Applications
-
Thermo-Physicochemical Properties
- Reaction engineering
- Chemical process design / simulation / optimization
- Energy efficiency improvement for combustion processes
- Chemical safety and regulation
-
Quantum Chemical Computation Data
- Optimized 3D molecular structure
- Energy level comparison among other molecules
- Speed up molecular optimization by starting from
the Mol-Instincts 3D structure
-
Molecular Descriptors
- Obtaining descriptor values without running software
- QSPR / QSAR modeling
-
Drug-Related Properties
- New drug discovery
- Drug possibility provision
-
Spectra Data
- Application study with IR / NMR / VCD
-
Quantum Chemical Property Data
- Obtaining optimized molecular structure (2D/3D)
- Vibrational frequency analysis & animation
- Molecular orbitals (HOMO, LUMO)
How to Use
Mol-Instincts Usage Guide
-
Access Mol-Instincts Search website.
https://search.molinstincts.com -
Search your compounds by Text / Structure / Property.
Search a Compound -
Click 'View our data' of the matching compound in the result list to move to the property view page.
View our data -
Similar compounds are also shown along with matching accuracy.
Results with Matching Accuracy -
Seven different property categories are available – simply select as needed.
View Chemical Properties
Accuracy
Accuracy Level of Above 95%
Due to the limitations of experiments, hundreds of estimation methods and software have been developed. These are, however, mostly based on empirical approaches whose accuracy is low.
The predicted data were compared and verified with millions of experimental data collected from every possible source, including journal, textbooks, and existing databases for more than 5 years.
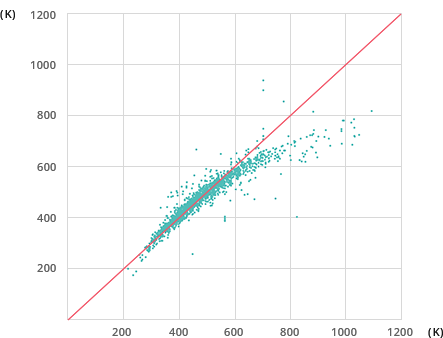
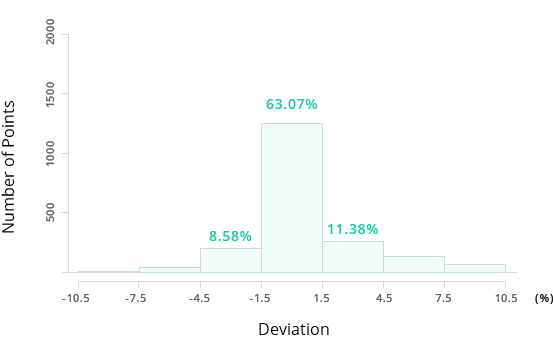
Accuracy of Existing Estimation Methods (Joback Method)
63.07%
Accuracy of Mol-Instincts Prediction
95.02%
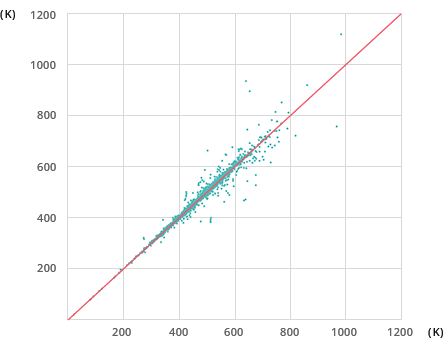
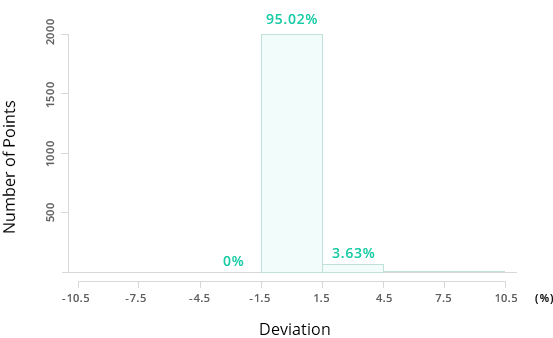
For the same example of normal boiling point estimation with 2,171 compounds, Mol-Instincts shows an accuracy level of above 95% while well-known existing Joback method shows accuracy of 63.07%
Development Process
40 Related Patents
-
01
High Quality
Quantum CalculationInput structure for the quantum calculation was determined by conformer analysis - where the most stable structure was used.
-
02
Most Advanced
QSPR ModelingQSPR modeling was performed with more than 2,000 molecular descriptors obtained from quantum chemical calculations.
-
03
Detailed Model
VerificationPredicted data were compared and verified with the experimental data available to date, where the accuracy level of 95% was confirmed in most cases.
-
04
Chemical Property
CategorizationThe Mol-Instincts database containing over 2,100 sets of data and information per compound was constructed.
Citation List
Cited in authoritative journals such as Nature
| PUBLISHER | PUBLICATION |
|---|
Patents List
| 2013.05.20 | Multiple Linear Regression―Artificial Neural Network Model Predicting Ideal Gas Absolute Entropy of Pure Organic Compound for Normal State |
|---|---|
| 2013.10.29 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Acentric Factor of Pure Organic Compound |
| 2013.10.29 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Critical Pressure of Pure Organic Compound |
| 2013.10.29 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Critical Temperature of Pure Organic Compound |
| 2013.10.29 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Critical Volume of Pure Organic Compound |
| 2013.10.29 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Energesis of Ideal Gas of Pure Organic Compound |
| 2013.10.29 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Enthalpy of Fusion at Melting Point of Pure Organic Compound |
| 2013.10.29 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Saturated Liquid Density of Pure rganic Compound at 298.15K |
| 2013.09.24 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Normal Boiling Point of Pure Organic Compound |
| 2013.09.24 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Refractive Index of Pure Organic Compound |
| 2013.05.20 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Solubility Index of Organic Compound |
| 2013.05.20 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Standard State Absolute Entropy of Pure Organic Compound |
| 2013.05.20 | Multiple Linear Regression―Artificial Neural Network Model Predicting Standard State Enthalpy of Formation of Pure Organic Compound |
| 2013.07.18 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Magnetic Susceptibility of Pure Organic Compound |
| 2013.08.21 | Multiple Linear Regression―Artificial Neural Network Model Predicting Polarizability of Pure Organic Compound |
| 2013.05.20 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Ionizing Energy of Pure Organic Compound |
| 2013.07.18 | Multiple Linear Regression Model Predicting Electron Affinity of Pure Organic Compound |
| 2013.08.09 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Parachor of Pure Organic Compound |
| 2013.08.21 | Multiple Linear Regression―Artificial Neural Network Model Predicting Flash Point of Pure Organic Compound |
| 2013.05.20 | Multiple Linear Regression- Artificial Neural Network Hybrid Model Predicting Lower Flammability Limit Temperature of Pure Organic Compound |
| 2013.08.06 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Lower Flammability Limit Volume Percent of Organic Compound |
| 2013.09.24 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Upper Flammability Limit Temperature of Organic Compound |
| 2013.08.21 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Upper Flammability Limit Volume Percent of Pure Organic Compound |
| 2013.05.20 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Liquid Density of Pure Organic Compound for Normal Boiling Point |
| 2013.09.24 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Heat of Vaporization of Pure Organic Compound for 298K |
| 2013.09.24 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Heat of Vaporization of Pure Organic Compound at Normal Boiling Point |
| 2013.08.06 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Octanol-Water Partition Coefficient of Pure Organic Compound |
| 2013.05.20 | Multiple Linear Regression-Artificial Neural Network Hybrid Model Predicting Water Solubility of Pure Organic Compound |
| 2013.04.23 | Multiple Linear Regression―Artificial Neural Network Hybrid Model Predicting Heat Capacity of Ideal Gas of Organic Compound |
| 2013.10.29 | SVRC Model Predicting Heat Capacity of Liquid of Pure Organic Compound |
| 2013.05.20 | SVRC Model Predicting Evaporation Heat of Pure Organic Compound |
| 2013.05.20 | SVRC Model Predicting Saturated Liquid Density of Pure Organic Compound |
| 2013.10.29 | QSPR Model Predicting Surface Tension of Liquid of Pure Organic Compound |
| 2013.08.27 | SVRC Model Predicting Thermal Conductivity of Liquid of Pure Organic Compound |
| 2013.08.06 | SVRC Model Predicting Thermal Conductivity of Gas of Pure Organic Compound |
| 2013.04.23 | SVRC Model Predicting Vapor Pressure of Liquid of Pure Organic Compound |
| 2013.09.24 | SVRC Model Predicting Liquid Viscosity of Pure Organic |
| 2013.09.24 | SVRC Model Predicting Gas Viscosity of Pure Organic |
| 2013.05.20 | Mathematical Model Predicting Second Virial Coefficient of Pure Organic Compound Through Boyle Temperature Prediction |
| 2013.05.02 | Automatic Method Using Quantum Mechanics Calculation Program and Materials Property Predictive Module and System therefor |
| 2014.03.12 | Method for Predicting a Property of Compound and System for Predicting a Property of Compound |